Pharmaceutical companies
Veeva Vault
Veeva Vault is an industry-leading, unified cloud platform designed exclusively for pharmaceutical, biotech, medical device, and life sciences organizations. It enables teams to manage regulated content, streamline quality processes, centralize regulatory operations, and ensure global compliance across the product lifecycle.
Veeva Vault: Complete Visibility & Compliance
Built specifically for the life sciences ecosystem, Veeva Vault delivers end-to-end visibility, faster workflows, and audit-ready documentation, making it one of the most trusted enterprise platforms for highly regulated industries.
Veeva Vault ensures full visibility, streamlined workflows, and audit-ready compliance for life sciences organizations.Why Veeva CRM Is the Trusted CRM for Pharmaceutical and Life Sciences Companies?
Enterprise-Grade Compliance
The platform is designed to meet global regulatory standards:
- FDA 21 CFR Part 11
- EMA and ICH guidelines
- GxP and GMP requirements
Every action is tracked through automated audit trails, ensuring full transparency for inspections.
AI-Enabled Efficiency and Automation
Veeva Vault integrates AI-powered automation to help teams:
- auto-organize content
- detect compliance risks
- accelerate review and approval cycles
- maintain consistent metadata/li>
Complete Ecosystem Integration
Veeva Vault connects seamlessly with
- Veeva CRM
- Veeva Quality Suite
- Veeva Regulatory Suite
- Veeva Commercial Cloud
- Veeva Medical Suite
This ensures that the right teams always access updated, approved, compliant content.
End-to-End Collaboration
Teams across regulatory, quality, medical, clinical, and commercial functions can collaborate in real time.
Unified Content and Data Management
Veeva Vault brings all documents, digital assets, quality records, regulatory files, and medical content together in one secure environment.
Tools and Modules Under Veeva Vault
Veeva Vault PromoMats
A compliant promotional content management system for MLR reviews, digital assets, and multi-channel promotional materials.
Veeva Vault MedComms
Designed for medical affairs teams to manage scientific content, FAQs, response documents, and medical communication workflows.
Veeva Vault QualityDocs
Controlled document management system (DMS) for SOPs, training documents, and quality procedures with full compliance.
Veeva Vault QMS
Manages quality processes including deviations, CAPAs, complaints, audits, and change control.
Veeva Vault RIM
End-to-end regulatory information management for submissions, labeling, registration tracking, and regulatory operations.
Veeva Vault Clinical
Supports clinical trials with eTMF, study documents, site files, and clinical trial workflows.
Veeva Vault Safety
Unified pharmacovigilance platform for safety case management, signal detection, and regulatory safety reporting.
Benefits of Veeva Vault for Pharmaceutical and Life Sciences Organizations
01. Single Source of Truth
Eliminates content duplication and outdated files by maintaining a centralized, compliant repository for all regulated content.
02. Faster Review and Approval Cycles
Automated workflows streamline content movement from creation to approval, significantly reducing review timelines.
03. Audit and Inspection Readiness
Every action is fully recorded, version-controlled, and traceable, ensuring continuous compliance and inspection readiness.
04. Global Visibility
Gain real-time visibility across regions, teams, and product portfolios for better decision-making and governance.
05. Scales Across the Entire Product Lifecycle
Supports R&D, clinical, regulatory, quality, medical, and commercial teams on a single unified platform.
Who Should Use Veeva Vault?
Veeva Vault is ideal for
-
-
Biotechnology organizations
-
Medical device manufacturers
-
Contract research organizations (CROs)
-
Contract manufacturing organizations (CMOs/CDMOs)
-
Life sciences service providers
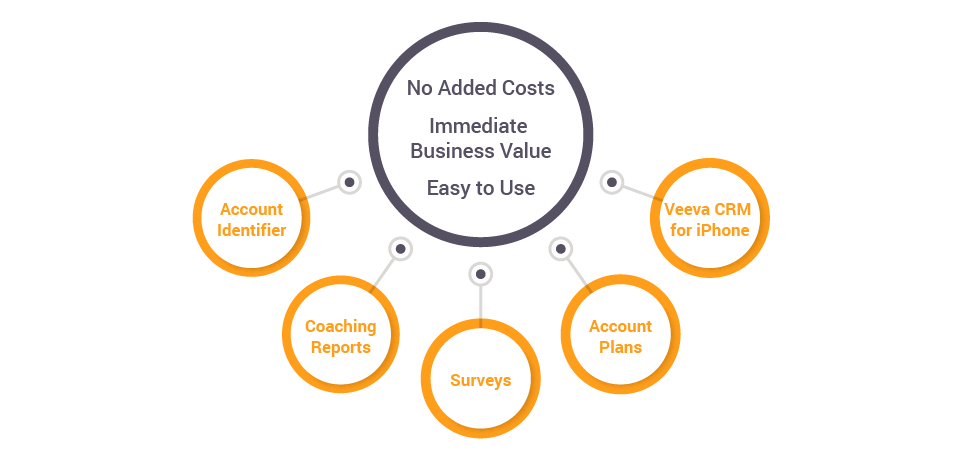

Why Choose Our Veeva Vault Solution Services?
We help you implement, customize, and optimize Veeva Vault to align with your workflows. Our expertise includes
-
System configuration
-
Workflow automation
-
Content migration
-
Integration with CRM and ERP systems
-
Training and user enablement
-
Compliance validation support
What Our Clients Say
Veeva Vault gave us complete control over our regulated content. All documents are easy to find, properly versioned, and always inspection ready. It has brought a lot of clarity to our daily work.
Our review and approval cycles became much faster after moving to Veeva Vault. Automated workflows reduced back and forth and helped teams focus more on quality instead of manual follow ups.
Content migration and system setup were handled very professionally. The solution was configured around our processes, which made user adoption much easier.
Different teams like quality, regulatory, and medical now collaborate smoothly in one system. Veeva Vault removed silos and improved transparency across departments.
Working with Cloud Rank on Veeva Vault was a great experience. They took time to understand our compliance needs and configured the system so we are always inspection ready.
David Kim
Founder
Fill out the form to connect with our experts
Looking to implement or optimize Veeva Vault for your organization?
Located in India
